
The medical devices undergo conformity assessment before they are included in the ARTG list to ensure that the devices comply with the essential principles required by the Therapeutic Goods Administration (TGA), Australia. Essential principles basically outline the safety and performance characteristics that any device should meet, so that it can be sold in Australia. Similar to the process in the EU, conformity assessment in Australia is based on the risk class of the device. Therefore, the conformity assessment route to be followed by any device depends on its classification.
There are various Regulatory standards which apply to various types of devices. Complying with either one or more of the standards is required as an essential requirement. Identification of relevant standards applicable to the device and subsequent testing of the device to demonstrate its compliance to the standard becomes a pre-requisite.
Conformity assessment involves a systematic examination of the technical documents concerned with the device. The risk management, clinical evaluation, manufacturing process involved, and the vigilance activities handled by the manufacturer are the critical areas for assessment. Devices that are required to have a TGA Conformity Assessment certificate are listed in Regulation 4.1 of the Therapeutic Goods (Medical Devices) Regulations 2002. While a conformity assessment certificate issued by the TGA is pre-requisite for marketing majority of medical devices in Australia, the TGA also accepts conformity assessment certificate issued by the Notified Bodies in Europe. In addition, the TGA also accepts conformity assessment from countries that are part of the MDSAP (Medical Device Single Audit Program).
The conformity assessment requirements vary with the risk category of the device. Table No.1 details the classification-based conformity assessment process in Australia.
Conformity Assessment Process - Australia
|
Class |
Conformity Assessment Procedure |
Manufacturer Responsibilities |
|
I |
Part 6 (Declaration of Conformity, not requiring assessment by the Secretary) |
Documentation to show conformity with Essential Principles |
|
I (measuring) & IIa (non-sterile) |
Part 6 (Declaration of Conformity, not requiring assessment by the Secretary) Part 5 (Product quality management system) |
Documentation to show conformity with Essential Principles. Implement product quality management system for final inspection and testing for audits. Note: Submission of Declaration of Conformity is not required for Class I (non- measuring and nonsterile) but should be available upon request by the TGA. |
|
I (sterile) & IIa (sterile) |
Part 6 (Declaration of Conformity, not requiring assessment by the Secretary) Part 4 (Production Quality Assurance) |
Documentation to show conformity with Essential Principles. Implement quality management system that excludes design element, based on ISO 13485. |
|
IIb |
Part 1 (Full Quality Assurance) excluding Clause 1.6 (Examination of design) |
Implement full quality management system including design, production, labeling, packaging and final inspection based on ISO 13485. Excludes design dossier. |
|
III & AIMD |
Part 1 (Full Quality Assurance) including Clause 1.6 (Examination of design) |
Implement full quality management system including design, production, labeling, packaging and final inspection based on ISO 13485. Design dossier in accordance with Essential Principles. |
|
Systems or Procedure Packs Part 7 |
(Procedures for Medical Devices Used for a Special Purpose) |
Essential Principles. Conformity Assessment procedure. Clinical evidence for individual component in system or pack. |
Table No.1. Conformity Assessment Requirements of the Devices
The usual approval process of medical devices by the TGA looks like:
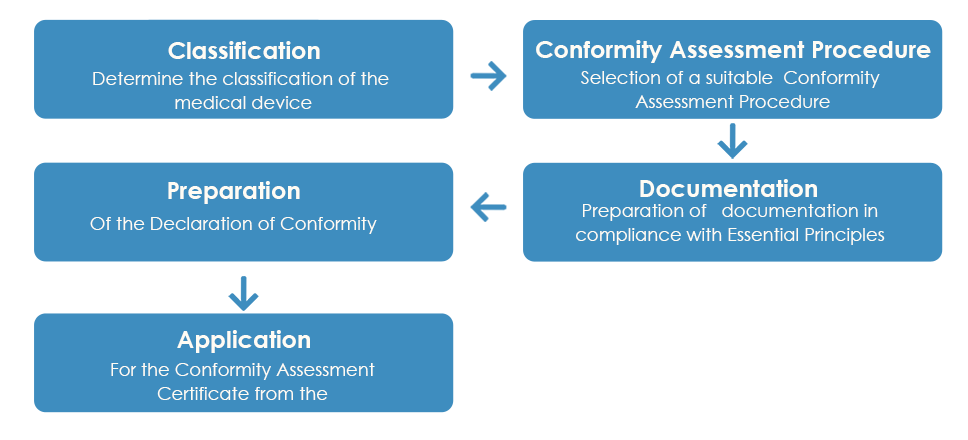
As detailed above, once the Conformity Assessment Certificate is received from the TGA, a Declaration of Conformity (DoC) needs to be prepared by the manufacturer declaring that the medical device complies with the applicable essential principles, classification rules and the conformity assessment route. However, unlike the Conformity Assessment Certificate, a European DoC is not accepted by the TGA.
The Australian market offers a promising landscape for medical device manufacturers, if the Regulatory requirements of the Therapeutic Goods Administration (TGA) are met., The conformity requirements and assessment process vary with risk class of device and IVD. Though the medical device regulations are well defined and transparent, navigating through them is complex and the manufacturers may seek support from the Regulatory partners for successful listing of the device.
To know comprehensive insights on the conformity assessment requirements, Australian Sponsor and ARTG listing in Australia, More contact a proven Regulatory expert. Stay informed. Stay compliant.
